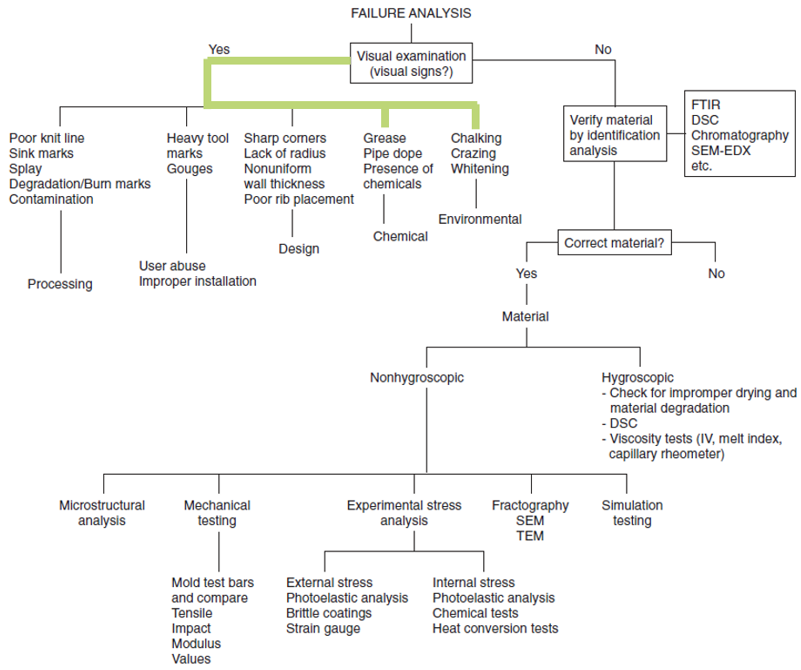
Root Cause Analysis of Plastic ESC
The ultrasound scan module in Verathon’s i10 bladder scanner contains mineral oil, which acts as a medium for signal transmission between the patient and the internal ultrasound transducer. Maintaining the integrity of the sealed oil enclosure is critical to the device’s performance. Therefore, it was concerning when several customer units were returned for service due to oil leakage. I was subsequently assigned to investigate this significant failure.
One morning, I arrived at the office to find several probes placed on my desk—delivered by our Service team for investigation into unexplained oil leaks. I immediately disassembled the probes to examine the scan module, the only sub-assembly that contains mineral oil. While no obvious damage was visible at first glance, closer inspection under a microscope revealed fine stress cracks around the O-ring groove of a PC/ABS injection-molded component. Enhancing the microscope images further exposed the full extent of the cracking, which explained the significant oil leakage observed in the returned units.
Starting out
Identifying the source of the oil leak was only the first step—it was a symptom, not the root cause. During disassembly of the scan module, I immediately noticed that the mineral oil had taken on a yellow hue, whereas it is typically clear. This discoloration suggested a chemical or environmental interaction affecting the mineral oil. Additionally, I noticed slight crazing on the surfaces of the PC/ABS component, specifically the areas that made contact with the discolored mineral oil. To investigate further, I followed the methodology outlined by Shah, V. (2021) in Handbook of Plastics Testing and Failure Analysis, which emphasizes that visual changes often indicate chemical or environmental degradation, such as environmental stress cracking (ESC).
Guided by this insight, I began analyzing the chemical composition of all 45 components within the scan module. Leveraging my background in chemistry, I designed an experiment to identify the specific compound responsible for the degradation. My first step involved comparing the substances used in each sub-component against known compatibility data for PC and ABS plastics, as documented in Woishnis’ Chemical Resistance of Thermoplastics (2012).
Reference:
Shah, V. (2021). Handbook of Plastics Testing and failure analysis. John Wiley & Sons, Inc. Pg 363-364.
Woishnis, W. A., & Ebnesajjad, S. (2012). Chemical Resistance of Thermoplastics. William Andrew Publishing.
The chemical compatibility evaluation of the scan module sub-components identified phthalate plasticizers as a likely cause of both the crazing of the PC/ABS plastic and the yellow discoloration in the mineral oil. These plasticizers are commonly used in nitrile rubbers, such as the Buna-N O-ring found in the sealing areas—precisely where the stress cracks occur. Notably, this O-ring is in direct contact with the PC/ABS component. Based on these observations, a failure theory was developed with the following sequence:
The scan module is assembled per assembly specifications - this assembly contains 3 nitrile rubber (Buna-N) O-rings.
Phthalate plasticizers migrate to the exterior surfaces of the nitrile rubber O-ring.
The mineral oil in the DCM Assembly serves a medium for diffusion of plasticizers, and therefore the plasticizers can migrate to all oil-contacting surfaces of the DCM Base. In the O-ring sealing areas, the O-ring makes direct contact with the plastic and therefore the plasticizers can directly diffuse into the plastic.
The phthalate plasticizers chemically attack the plastic and create localized weak areas and microscopic cracks.
Over time, the plastic experiences environmental stress cracking (ESC) and larger stress cracks are formed, which are eventually visible to the naked eye. This is also the point where oil leaks are observed.
Experiment Design
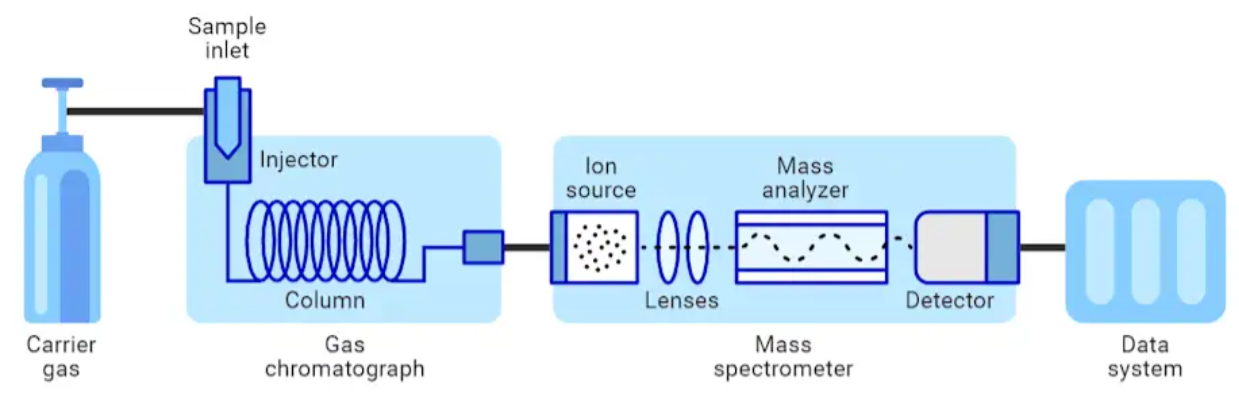
With a failure theory formulated, the next task was to provide evidence to prove/disprove the theory. First, an identification test was necessary to scan the assembly for phthalate plasticizers. Given that the mineral oil had visible changes in color, it made for an obvious choice in testing for substances that were not deliberately put into the scan module assembly. Gas chromatography mass spectrometry (GC-MS) was chosen as the preferred identification test since it provides sensitivity and selectivity required to find trace contaminants. Two identification tests needed to be performed:
One would identify phthalates in the yellow mineral oil – if this would be a negative result, then the phthalate theory could be immediately discredited.
The other would have verify the source of the phthalates – in this case, that the O-rings were indeed leeching plasticizers.
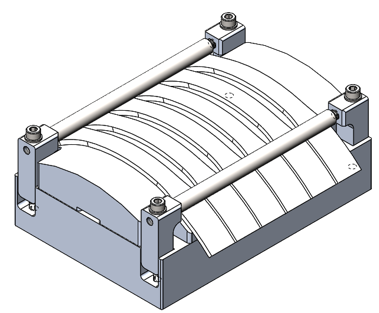
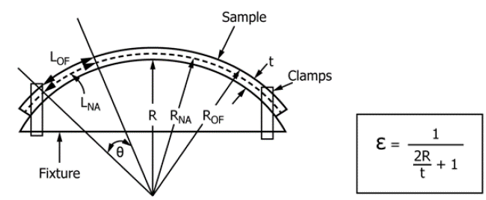
An important step in a root cause analysis is to be able to replicate or simulate the observed failure. This could be achieved by using controlled mixtures, material test strips, and a fixture to control strain/stress. A standard test for ESC in plastics is ASTM D543-21 and it allows the tester to simulate strain and chemical exposure under a controlled environment. Conditions were chosen such that they mirrored conditions in the scan module. A fixture was designed to meet the ASTM D543-21 standard, to hold six ASTM D638 Type I specimens, and to consistently apply a strain of 1.5% on all samples. This fixture could be used evaluate any material during ASTM D543-21 testing.
Once a solution for the failure is determined, then it would need to go through failure simulation testing to assess improvement. The acceptance criteria for the solution would be that the material tensile bar specimens do not show any stress cracking after being subjected to conditions from the failure simulation.
Results
This chromatogram compares new mineral oil from the manufacturing floor with samples from three scan modules containing yellowed oil. Four distinct peaks—absent in the new oil—indicate contaminants that diffused into the mineral oil. The most prominent peak, at around 11 minutes retention time, corresponds to dioctyl terephthalate (DOTP), a common plasticizer in nitrile rubbers. The remaining three, smaller peaks also represent phthalate plasticizers, differing in molecular structure by chain length and phenyl group positioning.
The identification test to verify the source of the DOPT was another GC-MS test on an extraction solution designed to extract chemical compounds from rubbers. ASTM D471-15a was used as the method for extraction using an n-Hexane solvent and our Buna-N O-rings. The chromatogram from the Buna-N O-ring extraction verifies that the DOPT was present and is therefore the source of the contaminant found in the failed scan modules assemblies with yellow mineral oil. Further, another peak of equal proportion can be seen, which corresponds to dibutoxyethoxyethyl adipate (another plasticizer with a commercial name of TP-95).
The failure simulation testing using the ASTM D543-21 method and strained PC/ABS specimens was successful in recreating the observation of stress cracks in the plastic. Both solutions with DOTP and TP-95 caused stress cracks in the PC/ABS specimens. It’s worth noting that TP-95 caused the most damage to the PC/ABS plastic, where distortion and etching of the surface occurred and the stress cracks extended completely through the thickness of the specimen.
The root cause analysis confirmed that the Buna-N O-ring was leaching plasticizers that caused environmental stress cracking (ESC) in the PC/ABS plastic. Since these plasticizers are known to degrade various plastics, the best solution was to replace the O-ring material. Viton O-rings were tested and found to contain no detectable plasticizers, making them an ideal substitute. Following their implementation in mid-2023, oil leak failures were fully resolved, with no incidents reported since.